ISSUE1585
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of Gvoke, a new formulation of injectable glucagon, for treatment of severe hypoglycemia.
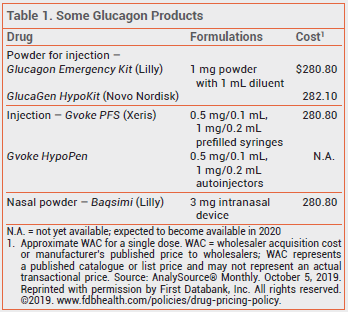
The FDA has approved a new formulation of glucagon (Gvoke – Xeris) for subcutaneous treatment of severe hypoglycemia in patients ≥2 years old with diabetes. Conscious patients with symptoms of hypoglycemia can take oral glucose. Glucagon is usually administered by a caregiver to an unresponsive patient. The new formulation is available in a single-use prefilled syringe (Gvoke PFS) and is expected to become available in a single-use auto-injector (Gvoke HypoPen) in 2020. Unlike previously available injectable glucagon products (Glucagon Emergency Kit, and others), Gvoke does not require reconstitution before administration. A glucagon nasal powder (Baqsimi) that does not require coordination with inhalation was recently approved for use in patients ≥4 years old.1
FDA approval of Gvoke was based on the results of two unpublished, randomized, crossover trials in a total of 161 adults and one single-arm trial in 31 children ≥2 years old with type 1 diabetes (available as abstracts and summarized in the package insert). Patients were given a continuous insulin infusion to reduce their blood glucose to <50 mg/dL (adult studies) or <80 mg/dL (pediatric study) and then treated subcutaneously with glucagon from Gvoke HypoPen or Glucagon Emergency Kit (all pediatric patients received Gvoke). In the adult studies, treatment success, defined as an increase in blood glucose of ≥20 mg/dL or to >70 mg/dL at 30 minutes after administration, was achieved in 99% of patients with Gvoke and in 100% with Glucagon Emergency Kit; the mean time to relief of symptoms in the two groups was similar.2,3 In the pediatric study, 100% of patients had an increase in blood glucose of ≥25 mg/dL, the primary endpoint.4 As with other glucagon formulations, the most common adverse effects of Gvoke were nausea and vomiting.
In a crossover ease-of-use trial, 14 of 16 adults successfully used Gvoke PFS under simulated real-world conditions; 5 successfully used Glucagon Emergency Kit.5 In two validation studies, 148 of 150 adults and adolescents (99%) successfully used Gvoke HypoPen or Gvoke PFS. The mean time required for preparation and administration was 60-70 seconds shorter with Gvoke than with Glucagon Emergency Kit.5,6
Children <12 years old weighing <45 kg should be given a 0.5-mg dose of Gvoke; all other patients should receive 1 mg. The drug should be injected subcutaneously into the lower abdomen, outer thigh, or outer upper arm. Emergency medical services should be called immediately after a dose is given. If there is no response to the first dose, a second dose can be given 15 minutes later.
- Glucagon nasal powder (Baqsimi) for severe hypoglycemia. Med Lett Drugs Ther 2019; 61:148.
- MP Christiansen et al. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the symptomatic relief of severe hypoglycemia. Presented at the American Diabetes Association's 78th Scientific Sessions, June 22-26, 2018, Orlando, FL. Available at: https://plan.core-apps.com. Accessed October 10, 2019.
- MP Christiansen et al. A phase 3 comparison of a novel liquid glucagon autoinjector to glucagon emergency kit for the treatment of severe hypoglycemia. Presented at the American Diabetes Association's 78th Scientific Sessions, June 22-26, 2018, Orlando, FL. Available at: https://plan.core-apps.com. Accessed October 10, 2019.
- B Buckingham et al. Liquid room temperature stable glucagon— glucose response in pediatric type 1 diabetes patients. Presented at the American Diabetes Association's 78th Scientific Sessions, June 22-26, 2018, Orlando, FL. Available at: https://plan.core-apps.com. Accessed October 10, 2019.
- V Valentine et al. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diabetes Technol Ther 2019; 21:522.
- B Newswanger et al. Human factors studies of a prefilled syringe with stable liquid glucagon in a simulated severe hypoglycemia rescue situation. Expert Opin Drug Deliv 2019; 16:1015.
