ISSUE1590
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Discuss the efficacy of tafamidis (Vyndaqel, Vyndamax) for treatment of transthyretin-mediated amyloid cardiomyopathy.
The FDA has approved tafamidis, an oral transthyretin stabilizer, in 2 different formulations (see Table 1) for treatment of adults with transthyretin-mediated amyloid cardiomyopathy (ATTR-CM). Tafamidis is the first drug to be approved in the US for this indication. Patisiran (Onpattro), a transthyretin-directed small interfering RNA, and inotersen (Tegsedi), a transthyretin-directed antisense oligonucleotide, were both recently approved for hereditary transthyretin amyloid polyneuropathy.

THE DISEASE — ATTR-CM is a progressive life-threatening disease caused by destabilization of the tetrameric transport protein transthyretin. When transthyretin tetramers dissociate, they form misfolded monomers that are deposited as amyloid fibrils in the myocardium, resulting in cardiomyopathy and progressive heart failure. Tafamidis selectively binds to and stabilizes transthyretin, preventing dissociation of the tetramers and slowing the formation of amyloid. There are 2 subtypes of the disease: hereditary ATTR-CM, which is caused by a mutation in the transthyretin gene, and the more common wild-type ATTR-CM, which is associated with aging, usually affecting men >60 years old.1,2
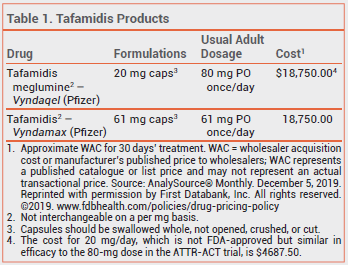
THE DRUG — Tafamidis is currently available in 2 formulations. Tafamidis meglumine (Vyndaqel) is available in 20-mg capsules; the dose is 80 mg (4 capsules) once daily. Tafamidis (Vyndamax) is available in 61-mg capsules; the FDA-approved dose is 61 mg (one capsule) once daily. The 80-mg dose of Vyndaqel and the 61-mg dose of Vyndamax produce similar serum concentrations.
CLINICAL STUDIES — In a randomized, double-blind, placebo-controlled, 30-month trial (ATTR-ACT) in 441 patients with hereditary or wild-type ATTR-CM, all-cause mortality rates (29.5% vs 42.9% with placebo) and cardiovascular-related hospitalization rates (0.48 per year vs 0.70 per year with placebo) were significantly lower with tafamidis meglumine 20 or 80 mg once daily; the two doses appeared to be similarly effective. The adverse effects of both doses were similar to those with placebo.3
The 80-mg dose of Vyndaqel was approved by the FDA because, in a pooled analysis of 11 studies, ex vivo stabilization of transthyretin tetramers was greater with the 80-mg dose than with the 20-mg dose; whether increased stabilization of transthyretin has clinical benefits is unclear.4
CONCLUSION — Tafamidis (Vyndaqel; Vyndamax) can decrease the number of hospitalizations and deaths in patients with transthyretin-mediated amyloid cardiomyopathy (ATTR-CM). Taking 20 mg of Vyndaqel daily appears to be as effective as 80 mg and costs much less. Long-term data on the comparative efficacy of the two doses are not available.
- MA Gertz et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 2015; 66:2451.
- FL Ruberg and JL Berk. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012; 126:1286.
- MS Maurer et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379:1007.
- FDA summary review. Vyndaqel (tafamidis meglumine). Available at http://bit.ly/2RO4s7F. Accessed December 16, 2019.
