ISSUE1631
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the data supporting the use of the adjuvanted, recombinant varicella zoster virus vaccine (Shingrix) in immunocompromised patients.
The FDA has licensed the adjuvanted, recombinant varicella zoster virus (VZV) vaccine Shingrix (GSK) for prevention of herpes zoster (shingles) in adults of any age who are or will be at elevated risk because of disease- or therapy-induced immunodeficiency or immunosuppression. Shingrix has been licensed for herpes zoster prevention in adults ≥50 years old since 2017.1 It is the only VZV vaccine currently available in the US; Zostavax, a live-attenuated VZV vaccine, was withdrawn from the market in 2020.
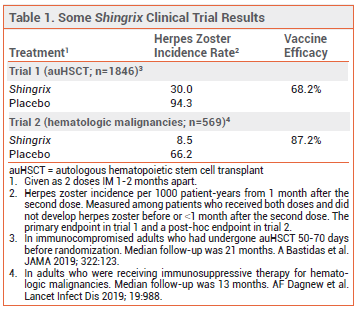
FDA licensure of Shingrix for the new indication was based on the results of two studies: a randomized, placebo-controlled trial in 1846 immunocompromised adults ≥18 years old who had received an autologous hematopoietic stem cell transplant within the previous 50-70 days, and a post-hoc analysis of a similar trial in 569 adults who were receiving immunosuppressive therapy for hematologic malignancies. In both trials, Shingrix significantly decreased the incidence of herpes zoster occurring ≥1 month after the second dose compared to placebo (see Table 1).2,3
Adverse effects of Shingrix in immunocompromised persons appear to be similar to those in healthy older adults. Myalgia, fatigue, headache, shivering, fever, GI symptoms, and injection-site pain, redness, and swelling are common. Severe local reactions preventing normal daily activities can occur.1,4 In a postmarketing observa-tional study in adults ≥65 years old, use of Shingrix was associated with an increased risk of Guillain-Barré syndrome in the 6 weeks after vaccination.5
In healthy older adults, Shingrix is typically given as two 0.5-mL doses administered intramuscularly 2-6 months apart. For immunocompromised patients who would benefit from a shorter vaccination schedule, the second dose can be given as early as 1 month after the first. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) on use of the vaccine in immunocompromised persons were not available at press time.
- Shingrix – an adjuvanted, recombinant herpes zoster vaccine. Med Lett Drugs Ther 2017; 59:195.
- A Bastidas et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 2019; 322:123.
- AF Dagnew et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019; 19:988.
- M López-Fauqued et al. Safety profile of the adjuvanted recombinant zoster vaccine in immunocompromised populations: an overview of six trials. Drug Saf 2021; 44:811.
- FDA Safety Communication. FDA requires a warning about Guillain-Barré Syndrome (GBS) be included in the prescribing information for Shingrix. March 24, 2021. Available at: https://bit.ly/3lmyvC6. Accessed August 3, 2021.