ONLY
ARTICLE
The FDA has approved Soaanz (Sarfez), a new formulation of the loop diuretic torsemide, for treatment of edema associated with heart failure or renal disease in adults. Torsemide has been available generically for years for treatment of hypertension and treatment of edema due to heart failure, renal disease, or hepatic disease. According to the manufacturer, Soaanz tablets are formulated to provide a gradual and sustained diuresis, lowering the risk of excessive urination and hypokalemia.1

STANDARD TREATMENT — Diuretics provide symptomatic relief of pulmonary and peripheral edema in patients with heart failure. Loop diuretics such as furosemide, bumetanide, and torsemide are more effective in patients with heart failure than thiazide-type diuretics such as hydrochlorothiazide. Torsemide and bumetanide have longer half-lives and are better absorbed than furosemide, but there is no clinical evidence that they are more effective.
CLINICAL STUDIES — No new clinical efficacy trials were required for FDA approval of Soaanz; approval was based on the established safety and efficacy of the original torsemide formulation and a pharmacokinetic/pharmacodynamic study in healthy volunteers showing that despite a lower relative bioavailability of Soaanz, the diuretic effect was similar to that of standard torsemide.1
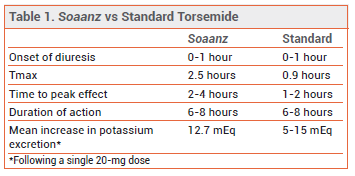
PHARMACOKINETICS — The peak serum concentration is lower and the time to peak concentration (Tmax) and effect are longer with Soaanz than with standard torsemide (see Table 1). Whether these differences decrease the risk of excessive urination remains to be established.
DOSAGE, ADMINISTRATION, AND COST — The initial dosage of Soaanz is 20 mg orally once daily; the dose should be doubled until desired diuresis is achieved. Thirty Soaanz 20-mg tablets costs $262, compared to $6.90 for generic torsemide.2
CONCLUSION — There is no evidence that Soaanz, the expensive new formulation of the oral loop diuretic torsemide, offers any clinical advantage over the original formulation.
- FDA. Center for Drug Evaluation and Research. Summary review. Soaanz. June 14, 2021. Available at: https://bit.ly/4cfitRe. Accessed August 7, 2024.
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. August 5, 2024. Reprinted with permission by First Databank, Inc. All rights reserved. ©2024. www.fdbhealth.com/policies/drug-pricing-policy.